We use cookies to understand how you use our site and to improve your experience.
This includes personalizing content and advertising.
By pressing "Accept All" or closing out of this banner, you consent to the use of all cookies and similar technologies and the sharing of information they collect with third parties.
You can reject marketing cookies by pressing "Deny Optional," but we still use essential, performance, and functional cookies.
In addition, whether you "Accept All," Deny Optional," click the X or otherwise continue to use the site, you accept our Privacy Policy and Terms of Service, revised from time to time.
You are being directed to ZacksTrade, a division of LBMZ Securities and licensed broker-dealer. ZacksTrade and Zacks.com are separate companies. The web link between the two companies is not a solicitation or offer to invest in a particular security or type of security. ZacksTrade does not endorse or adopt any particular investment strategy, any analyst opinion/rating/report or any approach to evaluating individual securities.
If you wish to go to ZacksTrade, click OK. If you do not, click Cancel.
Pharma Stock Roundup: Pfizer's Velsipity Receives EU Nod, ABBV Gets New CEO & More
Read MoreHide Full Article
This week, Pfizer’s (PFE - Free Report) Velsipity (etrasimod), an ulcerative colitis (UC) pill, was approved in the European Union (EU). AbbVie (ABBV - Free Report) announced that Robert A. Michael will be its new chief executive officer (CEO), replacing Richard A. Gonzalez, effective Jul 1, 2024. AstraZeneca (AZN - Free Report) and Merck (MRK - Free Report) announced regulatory updates by the FDA.
Recap of the Week’s Most Important Stories
EU Approval for Pfizer’s Velsipity: The European Commission granted marketing approval to Pfizer’s oral, once-daily pill called Velsipity to treat moderately to severely active UC. The oral, once-daily pill for UC, a chronic condition, became the only advanced therapy approved for use in patients 16 years of age or older in the EU.
The approval was expected in December 2023 as the European Medicines Agency’s Committee for Medicinal Products for Human Use had given a positive opinion recommending Velsipity’s approval. The FDA had approved Velsipity for UC in October 2023. Velsipity’s approval was based on data from two pivotal phase III studies, ELEVATE UC 52 and ELEVATE 12.
AbbVie Gets a New CEO: AbbVie announced the promotion of Robert A. Michael, its current president and chief operating officer, to the position of CEO. Robert Michael will succeed Richard A. Gonzalez, who is retiring from his role as CEO after serving in this capacity ever since AbbVie was formed in 2013. AbbVie came into existence on Jan 1, 2013, after Abbott Laboratories divested its pharmaceutical division. Gonzalez will become executive chairman of the board of directors, effective Jul 1, 2024, when the transition occurs. Robert Michael has also been appointed as a member of the board of directors.
AbbVie announced a collaboration with Tentarix Biotherapeutics to develop novel multifunctional biologics against one target in oncology and another in immunology. Once Tentarix nominates candidates, leveraging its proprietary Tentacles platform, AbbVie will receive an exclusive option to acquire the two therapeutic programs. As part of the deal, AbbVie will make upfront option payments totaling $64 million to Tentarix for the two programs.
FDA Approves AstraZeneca’s Tagrisso+Chemo for EGFR-mutated advanced NSCLC: AstraZeneca announced that the FDA has granted approval to Tagrisso (osimertinib) in combination with chemotherapy for treating EGFR-mutated advanced lung cancer. The approval was based on data from the FLAURA2 study, which showed Tagrisso plus chemotherapy extended median progression-free survival by nearly nine months versus standard-of-care treatment. Tagrisso is, at present, approved as a monotherapy for first-line treatment of locally advanced or metastatic EGFRm non-small cell lung cancer (NSCLC), locally advanced or metastatic EGFR T790M mutation-positive NSCLC and adjuvant treatment of early-stage EGFRm NSCLC.
AstraZeneca and Daiichi Sankyo announced that the FDA had accepted its biologics license application (BLA) seeking approval of its antibody-drug conjugate, datopotamab deruxtecan (Dato-DXd), for treating locally advanced or metastatic nonsquamous NSCLC in adult patients who have received prior systemic therapy. The FDA is expected to give its decision on the BLA in the fourth quarter of 2024 as the BLA was granted a standard review.
The BLA is based on data from the TROPION-Lung01 phase III study evaluating Dato-DXd in patients with HR-positive, HER2-low or negative breast cancer.
FDA Grants Priority Tag to Merck’s Keytruda sBLA in Endometrial Carcinoma: Merck announced that the FDA has granted priority review status to a new supplemental BLA (sBLA) seeking approval for Keytruda plus chemotherapy for treating primary advanced or recurrent endometrial carcinoma. The sBLA is based on data from the from the pivotal phase III NRG-GY018 study. The FDA is expected to give its decision on the label expansion on Jun 21, 2024.
In this study, Keytruda plus chemotherapy (carboplatin and paclitaxel) achieved a statistically significant and clinically meaningful improvement in progression-free survival versus chemotherapy in the abovementioned mentioned patient group, regardless of mismatch repair status. In the United States, at present, Keytruda is approved for two indications of endometrial carcinoma.
The NYSE ARCA Pharmaceutical Index rose 1.8% in the last five trading sessions.
Image: Bigstock
Pharma Stock Roundup: Pfizer's Velsipity Receives EU Nod, ABBV Gets New CEO & More
This week, Pfizer’s (PFE - Free Report) Velsipity (etrasimod), an ulcerative colitis (UC) pill, was approved in the European Union (EU). AbbVie (ABBV - Free Report) announced that Robert A. Michael will be its new chief executive officer (CEO), replacing Richard A. Gonzalez, effective Jul 1, 2024. AstraZeneca (AZN - Free Report) and Merck (MRK - Free Report) announced regulatory updates by the FDA.
Recap of the Week’s Most Important Stories
EU Approval for Pfizer’s Velsipity: The European Commission granted marketing approval to Pfizer’s oral, once-daily pill called Velsipity to treat moderately to severely active UC. The oral, once-daily pill for UC, a chronic condition, became the only advanced therapy approved for use in patients 16 years of age or older in the EU.
The approval was expected in December 2023 as the European Medicines Agency’s Committee for Medicinal Products for Human Use had given a positive opinion recommending Velsipity’s approval. The FDA had approved Velsipity for UC in October 2023. Velsipity’s approval was based on data from two pivotal phase III studies, ELEVATE UC 52 and ELEVATE 12.
AbbVie Gets a New CEO: AbbVie announced the promotion of Robert A. Michael, its current president and chief operating officer, to the position of CEO. Robert Michael will succeed Richard A. Gonzalez, who is retiring from his role as CEO after serving in this capacity ever since AbbVie was formed in 2013. AbbVie came into existence on Jan 1, 2013, after Abbott Laboratories divested its pharmaceutical division. Gonzalez will become executive chairman of the board of directors, effective Jul 1, 2024, when the transition occurs. Robert Michael has also been appointed as a member of the board of directors.
AbbVie announced a collaboration with Tentarix Biotherapeutics to develop novel multifunctional biologics against one target in oncology and another in immunology. Once Tentarix nominates candidates, leveraging its proprietary Tentacles platform, AbbVie will receive an exclusive option to acquire the two therapeutic programs. As part of the deal, AbbVie will make upfront option payments totaling $64 million to Tentarix for the two programs.
FDA Approves AstraZeneca’s Tagrisso+Chemo for EGFR-mutated advanced NSCLC: AstraZeneca announced that the FDA has granted approval to Tagrisso (osimertinib) in combination with chemotherapy for treating EGFR-mutated advanced lung cancer. The approval was based on data from the FLAURA2 study, which showed Tagrisso plus chemotherapy extended median progression-free survival by nearly nine months versus standard-of-care treatment. Tagrisso is, at present, approved as a monotherapy for first-line treatment of locally advanced or metastatic EGFRm non-small cell lung cancer (NSCLC), locally advanced or metastatic EGFR T790M mutation-positive NSCLC and adjuvant treatment of early-stage EGFRm NSCLC.
AstraZeneca and Daiichi Sankyo announced that the FDA had accepted its biologics license application (BLA) seeking approval of its antibody-drug conjugate, datopotamab deruxtecan (Dato-DXd), for treating locally advanced or metastatic nonsquamous NSCLC in adult patients who have received prior systemic therapy. The FDA is expected to give its decision on the BLA in the fourth quarter of 2024 as the BLA was granted a standard review.
The BLA is based on data from the TROPION-Lung01 phase III study evaluating Dato-DXd in patients with HR-positive, HER2-low or negative breast cancer.
FDA Grants Priority Tag to Merck’s Keytruda sBLA in Endometrial Carcinoma: Merck announced that the FDA has granted priority review status to a new supplemental BLA (sBLA) seeking approval for Keytruda plus chemotherapy for treating primary advanced or recurrent endometrial carcinoma. The sBLA is based on data from the from the pivotal phase III NRG-GY018 study. The FDA is expected to give its decision on the label expansion on Jun 21, 2024.
In this study, Keytruda plus chemotherapy (carboplatin and paclitaxel) achieved a statistically significant and clinically meaningful improvement in progression-free survival versus chemotherapy in the abovementioned mentioned patient group, regardless of mismatch repair status. In the United States, at present, Keytruda is approved for two indications of endometrial carcinoma.
The NYSE ARCA Pharmaceutical Index rose 1.8% in the last five trading sessions.
Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
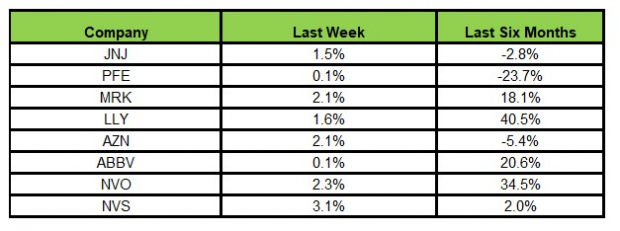
In the last five trading sessions, all the stocks were in the green, with Novartis rising the most (3.1%).Image Source: Zacks Investment Research
In the past six months, Lilly has risen the most (40.5%), while Pfizer has declined the most (23.7%).
(See the last pharma stock roundup here: AZN & LLY’s Q4 Earnings, NVO, NVS & MRK’s M&A Deals)
What's Next in the Pharma World?
Watch for regular pipeline and regulatory updates next week.